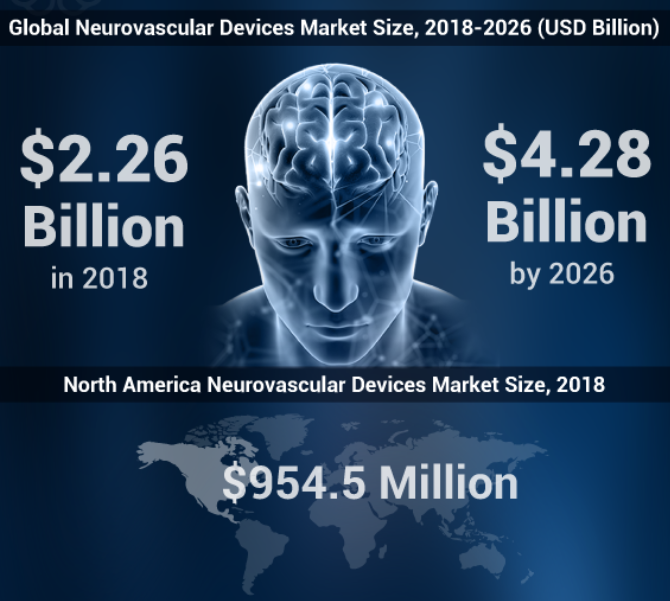
The global “neurovascular devices market” size is estimated to reach USD 4.28 billion by 2026, exhibiting a CAGR of 8.3% during the forecast period. One of the key drivers propelling the growth of this market is the increasing incidence of strokes on a global scale. According to the Brain Aneurysm Foundation, an estimated 6 million Americans each year suffer from cerebral aneurysms. The condition is also responsible for a half-million deaths globally each year. About 40% of ruptures result in death, and of those who survive, approximately 66% have some permanent brain damage. These neurovascular devices market trends along with the accessibility of new treatment options will substantively raise the demand for neurovascular devices in the forthcoming years.
As per the report, published by Fortune Business Insights, titled “Neurovascular Devices Market Size, Share & Industry Analysis, By Device Type (Stenting Systems, Embolization Devices, Neurothrombectomy Devices and Support Devices), By Application (Cerebral Aneurysms, Ischemic Stroke and Others), By End User (Hospitals, Specialty Clinics and Others) and Regional Forecast, 2019-2026” the market stood at USD 2.26 billion in 2018. The report stresses on all the aspects and characteristics of the market. It offers superior insights and shares cumulative data of all the segments. It is brought together after extensive research followed by deep analysis to benefit companies, stakeholders, financers and potential investors. It is designed with a motive to give a brief overview of the market, which involves the latest market trends, new advancements, product launches and any other recent activity that took place in the market.
Increasing Reimbursement Policies to Enrich Market Potential
The government is implementing strict regulations for devices, followed by the implementation of reimbursement policies for the treatment of neurovascular diseases. This is solely due to the rising cases of strokes, which is burdensome for the patients on account of the high-cost associated with treatment. In the U.S., the average cost per person for treatment of ischemic stroke is estimated to be around USD 73,000 to USD 77,300 annually. Therefore, the increasing availability of reimbursement through public and private health insurance has created a vast space for the expansion of the neurovascular devices market growth. Therefore, an increasing number of patients undergoing treatment and surgeries in the emerging nations has impelled the government and non- government organizations to take stern decisions for the implementation of reimbursement policies.
Click here to get the short-term and long-term impact of COVID-19 on this market.
Please visit: https://www.fortunebusinessinsights.com/industry-reports/neurovascular-devices-interventional-neurology-market-101684
Numerous Advantages of Embolization Devices to Invoke Demand
The neurosurgeons are primarily focusing on the embolization of intracranial haemorrhages and cerebral aneurysms. The durability of the embolization devices over surgical clipping procedures has proven to be one of the major contributing factors in fuelling the demand for embolization devices. The superiority of the device in demonstrating clinical efficiency will augment healthy growth for the segment. Additionally, the aim to create cost-effective and efficient devices by key players will substantively have a positive impact on the neurovascular devices market shares.
High-Cost Treatment Options to Dampen Growth
The expensive cost associated with the treatment of strokes is expected to hamper the neurovascular devices market growth. For instance, in the U.S, the economic cost burden of strokes is estimated to be over USD 30.0 Billion annually, including direct and indirect medical costs. Moreover, lack of awareness regarding strokes and neurovascular conditions among patients in the emerging nations will further aggravate the growth of the neurovascular devices/interventional neurology.
Some of the Prominent Players Present in the Global Neurovascular Devices Market
- Medtronic
- Penumbra, Inc.
- Stryker
- Terumo Corporation
- Johnson & Johnson Services, Inc.
- Merit Medical Systems
- L. Gore & Associates, Inc.
- MicroPort Scientific Corporation
- Other Players